And of course you could redo this calculation to find the volume of 1 mole of an ideal gas at room temperature and pressure or any other temperature and pressure.
Room temperature and pressure volume of gas.
Charles s law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant.
Satp standard ambient temperature and pressure is a reference with temperature of 25 o c 298 15 k and pressure of 101 325 kpa.
59 00 f and 101 325 kpa 1 00 atm.
Ideal gas law relation between the pressure volume amount and temperature of a gas under conditions derived by combination of the simple gas laws standard conditions of temperature and pressure stp 273 15 k 0 c and 1 atm 101 325 kpa standard molar volume volume of 1 mole of gas at stp approximately 22 4 l for gases behaving ideally.
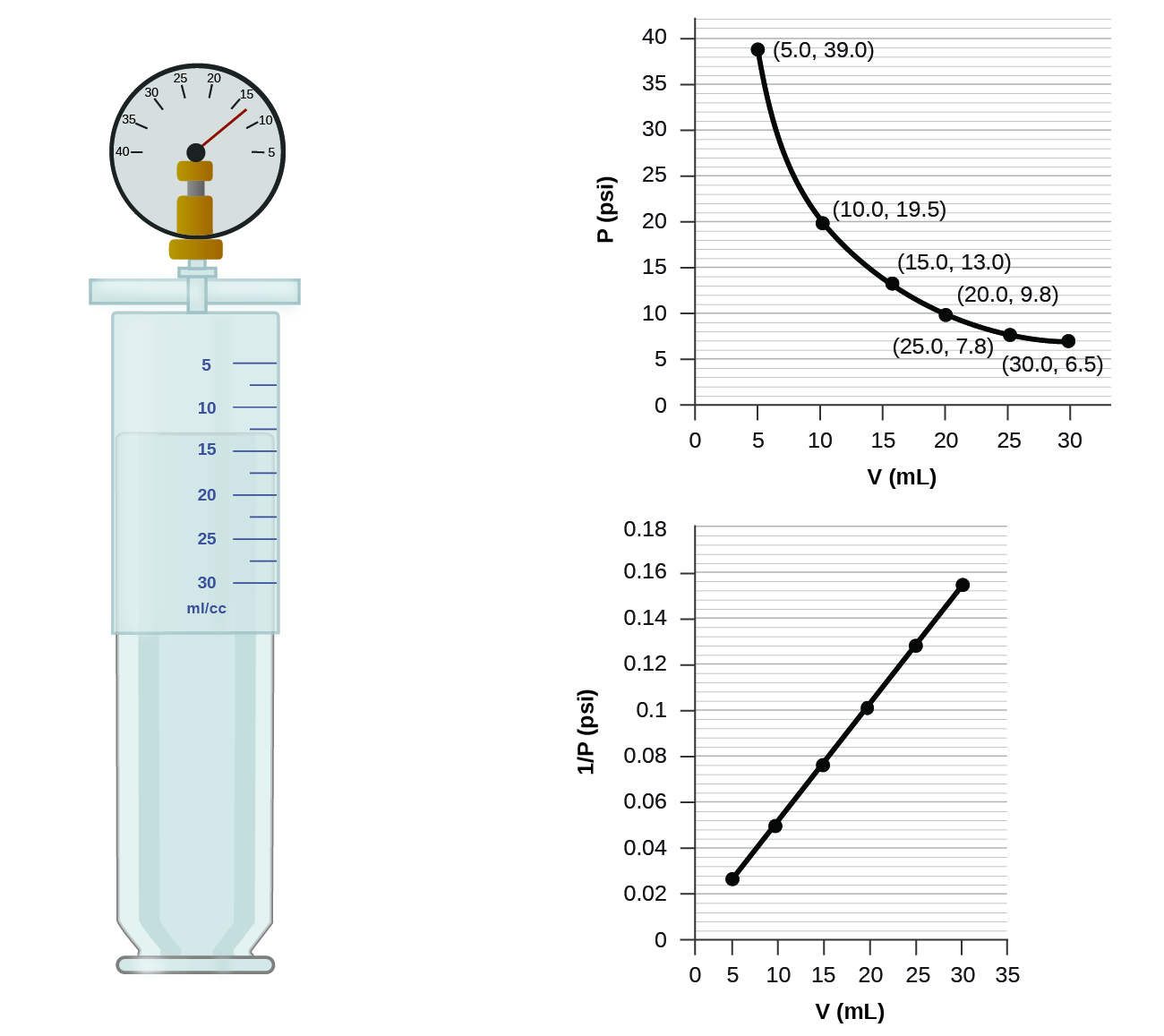
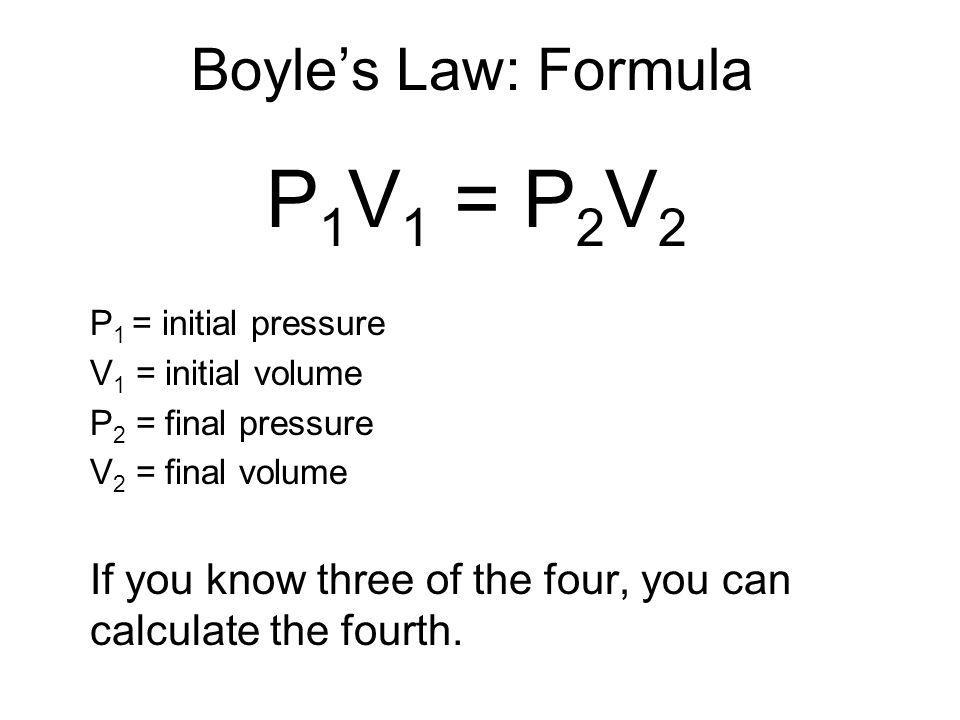
Equations explain the relationship between pressure temperature and volume in gases.
Ideal gas law calculator.
This volume is called the molar volume of a gas.
Same as before a constant can be put in.
1 if the volume of a container is increased the temperature increases.
The temperature volume law jacques charles 1746 1823 this law states that the volume of a given amount of gas held at constant pressure is directly proportional to the kelvin temperature.
Temperature can be measured using the celsius and kelvin scales.
Charles law gives the relationship between volume and temperature if pressure and amount of gas are held constant.
Easily calculate the pressure volume temperature or quantity in moles of a gas using this combined gas law calculator boyle s law calculator charles s law calculator avogadro s law calculator and gay lussac s law calculator in one supports a variety of input metrics such as celsius fahrenheit kelvin pascals bars atmospheres and volume in both metric and.
French physicist jacques charles 1746 1823 studied the effect of temperature on the volume of a gas at constant pressure.
2 if the volume of a container is decreased the temperature decreases.
As the volume goes up the temperature also goes up and vice versa.
This means that the volume of a gas is directly proportional to its temperature.
Satp standard ambient temperature and pressure is also used in chemistry as a reference.
This equation shows how the volume of gas in dm.
At these conditions the volume of 1 mol of a gas is 24 4651 liters.
Satp standard ambient temperature and pressure.
The absolute temperature is temperature measured with the kelvin scale.
V t c.
This is about as tricky as it gets using the ideal gas.
760 torr during those same years the most commonly used standard reference conditions for people using the imperial or u s.
Before 1918 many professionals and scientists using the metric system of units defined the standard reference conditions of temperature and pressure for expressing gas volumes as being 15 c 288 15 k.
The molar volume of an ideal gas is therefore 22 4 dm 3 at stp.